At 32 years old, I was given 16 months to live. On an ordinary Tuesday morning in November, an ultrasound detected 12 lesions on my liver that would soon be confirmed as stage 4, incurable ocular melanoma.
It wasn’t supposed to happen. Just one year earlier, I had become a cancer survivor in 22 days. A single tumour was contained in my eyeball and a biopsy of the cells gave me the best possible outcome: There was a less than 2% chance of metastatic disease.
Advertisement
But somebody has to be the 2%.
My oncologist said I had one FDA-approved treatment option, and I trusted her. I didn’t have time to get a second opinion. In the 16 months she predicted I had left if I agreed to the treatment she suggested, I needed to get my affairs in order and explain to my precious nephews and niece why their (favourite) auntie wouldn’t live to see them get their drivers’ licenses, graduate or get married.
As he’d already been planning to do, my boyfriend Nick proposed to me on Thanksgiving, two days after my diagnosis. I had expected to spend the day in the corner of the room brooding over my fateful news and avoiding loved ones. His proposal and my new ring swept me up in a wave of hope and optimism for a future I wasn’t sure existed.

Courtesy of Katie Ortman Doble
And while I vacillated between planning my funeral and my wedding in my head, I had one crucial advantage in my new reality: My father is a doctor of internal medicine.
Advertisement
Before a drug becomes FDA-approved, it goes through the clinical trial process. For someone like me with an advanced, rare disease, clinical trials offered a better shot at buying more time, which is really all we’re hoping to do.
My father asked my oncologist about clinical trials.
“That would be very expensive,” was all she told us.
We, rightfully so, give doctors authority. They have advanced degrees. They dedicate their lives to understanding human biology, science and pharmaceuticals. I’m just a headhunter. What do I know?
If it were not for my father, who questioned the authority of this doctor, I would have blindly followed her. And I can say with certainty that had I done that, I would be dead right now.

Courtesy of Katie Ortman Doble
My father, whom I began referring to as my agent, spent every waking moment talking to doctors all over the country to find a glimmer of hope — something that could buy us more than 16 months.
Advertisement
He got me an appointment that December at Memorial Sloan Kettering in New York City. And so began my crash course in clinical trials (also called studies or protocols). My “agent,” fiancé (a word that tied me to a future in which I was alive) and I met with the doctor there, who explained our options.
He spoke about the importance of strategy in creating my treatment plan. Had I started on the first treatment option provided by the oncologist who diagnosed me, for example, I would not have been eligible for the clinical trial he was running.

Courtesy of Katie Ortman Doble
I have heard of other patients who frantically start chemotherapy upon a terminal diagnosis, only to find that made them ineligible for potentially life-saving clinical trials. When you’re given an expiration date, it’s hard to take a pause on attacking the thing that is attacking you, but this approach can mean having a matter of months versus years (or life and death).
All cancer treatments start as clinical trials, so clinical trials are essential in advancing medicine. The clinical trial process typically includes four phases focused on safety, efficacy, comparison to standard of care and the safety of treatment over time, and the majority of drugs fail in one of these phases. The estimated median cost to develop just one successful drug is $1 billion and approval takes 12 years on average. The FDA approval process itself takes six to 10 months.
Advertisement
The first five weeks of the MSK trial were packed with appointments, and as a Midwest girl living in Denver, I was secretly thrilled with the opportunity to temporarily live in a tiny, expensive apartment in Midtown Manhattan. While I worked in my company’s Midtown office, I also saw a Broadway show, went wedding dress shopping and found myself with a hideous, full-body rash from the trial’s side effects the week before my wedding.

Courtesy of Katie Ortman Doble
I was starting to understand the expense of clinical trials that my first doctor referenced. For me, the financial burden came from the airfare and lodging. I’ve been lucky to have excellent insurance through work and a job that allows me the time off. I have peers who have survived cancer only to be left six figures in debt, which leads to crippling stress, which in turn damages their health.
Those who don’t have insurance, or have astronomical deductibles, or can’t afford the financial demands that come with participating in a trial, aren’t able to get treatment that could save their lives. It isn’t fair that our health care system favours the privileged.
I spent eight months in treatment at MSK, flying to NYC monthly from Denver to pick up my medication and get scans. But by August of that year, the growth of my tumours eliminated me from the trial. Thankfully, we were ready with the next trial option.
Advertisement
This was another critical strategy introduced by my doctor at MSK that I encourage other patients to explore and understand. For someone like me, it wasn’t likely that I’d only do one clinical trial. While my doctor kept my tumours at bay, he and my dad continued conversations with doctors all over the country to be sure we had a plan B and C, in case I wasn’t eligible for plan B. We didn’t want to wait until I was eliminated from my first trial. We wanted to be ready. And since some trials and treatments might make me ineligible for others in the future, we had to be very methodical with our next step.
I participated in two trials back home in Denver over the next two years while also exploring Y90 or liver embolisation, something that was only FDA-approved for metastatic colon cancer at the time. My tumours responded really well to that therapy, buying me 3.5 years of stability and the chance to finally enjoy a delayed honeymoon with my husband.

Courtesy of Katie Ortman Doble
In May 2020, substantial growth in my tumours pushed me into my fourth clinical trial at UPMC-Pittsburgh. Getting into clinical trials isn’t guaranteed or easy, especially when cross-country travel is involved. It typically includes a battery of tests like an EKG, echocardiogram and a CT scan or MRI. In my case, I had three liver biopsies in short order from three separate hospitals because each wanted its own tissue.
Liver biopsies feel like someone is taking a staple gun to your liver. Our bodies endure so much simply staving off cancer, not to mention the pokes and prods that come from treatment. The doctor also needs to see that you and your bloodwork are within the safety parameters of the trial.
Advertisement
Just as getting in isn’t guaranteed or easy, neither is staying in the trial. When you get eliminated from a trial, it’s usually for tumour growth or something else entirely out of your control. I was unknowingly kicked out of my second trial after one infusion because I was taking steroids that the doctor prescribed to combat one of the side effects. I was grateful that I continued to have options, but the volatility of it all was exhausting. The worst part was always the in between, when I didn’t have a plan and the fear of the next treatment haunted me.
In Pittsburgh, the goal of the TIL therapy was to re-sculpt my immune system with cancer-fighting cells. Enrolling was labour-intensive. There were the standard exams that deemed me eligible, plus the steps involved to create my treatment. The doctor laparoscopically removed two tumours from my liver and from those cells, grew the TIL or Tumour Infiltrating Lymphocytes. A leukapheresis extracted my white blood cells, which were then fed to the TIL, multiplying them from the millions to the billions.
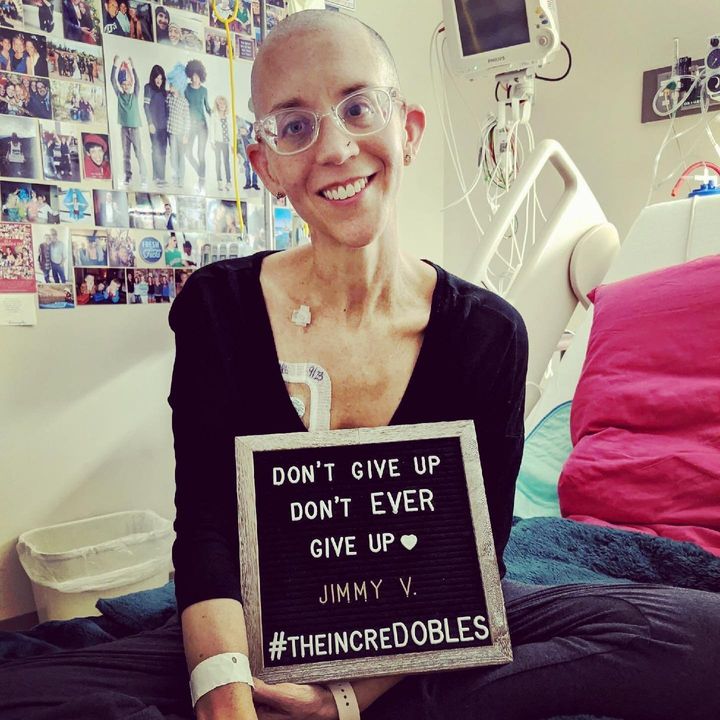
Courtesy of Katie Ortman Doble
That September, I spent three weeks in the hospital receiving seven days of chemotherapy, my TIL infusion and IL-2. Because of that one time I was unexpectedly eliminated from a trial, I held my breath until the TIL hit my bloodstream. I closed my eyes and pictured the reprogrammed cells reuniting with their former tumour friends to now attack them.
I decorated the muted coloured walls of my hospital room like a dorm with my “F*CK cancer” cross-stitch, pictures of my nephews, nieces and loved ones, and pictures of my body doing adventurous things like skydiving, kayaking and jumping into the Adriatic Sea. When my hair started to fall out, Nick shaved my head and went back to the hotel to shave his own.
Advertisement
My life started to feel like a game of whack-a-mole. But I was so grateful. Through all the flying back and forth, different doctors and side effects, I was buying time I wouldn’t have had if I’d listened to my first oncologist. At the time of my diagnosis, there was only a half page of trials to explore. When I had growth in 2020, there were over three pages.
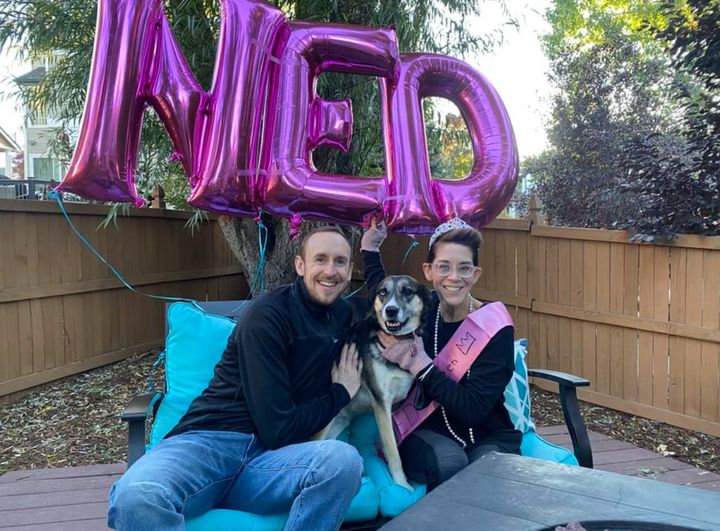
Courtesy of Katie Ortman Doble
I responded well to TIL, with some tumours shrinking and a few disappearing. One rogue tumour that my doctor called the “festering problem” kept growing. Naturally, I named it Uncle Fester. In September of 2021, my doctor told me he wanted to surgically remove Uncle Fester and all remaining cancer, and I eagerly obliged.
On Sept. 17, 2021, nearly seven years after I’d been given 16 months to live, I awoke from surgery to hear the words we once thought impossible: “We got it all, Katie. You are no evidence of disease (NED).”
If it weren’t for my father/agent, I wouldn’t have known how to navigate the world of clinical trials that ultimately bought me enough time to (so far) see the first of nine nephews and nieces drive his car.
Advertisement
Clinical trials are not going to be the answer for every diagnosis, but they need to be a consideration, not an afterthought. If you or a loved one gets diagnosed with cancer, talk to your doctor about what clinical trials are available. This might require getting a second opinion — which ideally is done prior to starting any treatment.

Courtesy of Katie Ortman Doble
This month marks 20 months since I became NED. My doctor continues to monitor me, and I am cautiously hopeful. Of course, there’s the chance my cancer returns. My doctor hopes that the TIL in my bloodstream remains dormant in my lymph nodes and reactivates if that happens.
I haven’t had a reason to speak to my original oncologist since seeking a second opinion, but I’m almost certain she thinks I’m dead.
Katie Ortman Doble is a headhunter, stage 4 cancer survivor, patient advocate, keynote speaker and author (blog: Future Happy Self) who is currently seeking an agent for her memoir. In 2017, Katie was given the Courage Award from the Melanoma Research Foundation. She received the First Descents′ Out Living It Award in 2021 and was recently highlighted as a Medical Hero by CISCRP. She and her husband, Nick, reside in Denver with their doghter, Alice. Connect on Liinks and follow Katie’s story of survival on Instagram/Twitter @ceortman.
Advertisement











